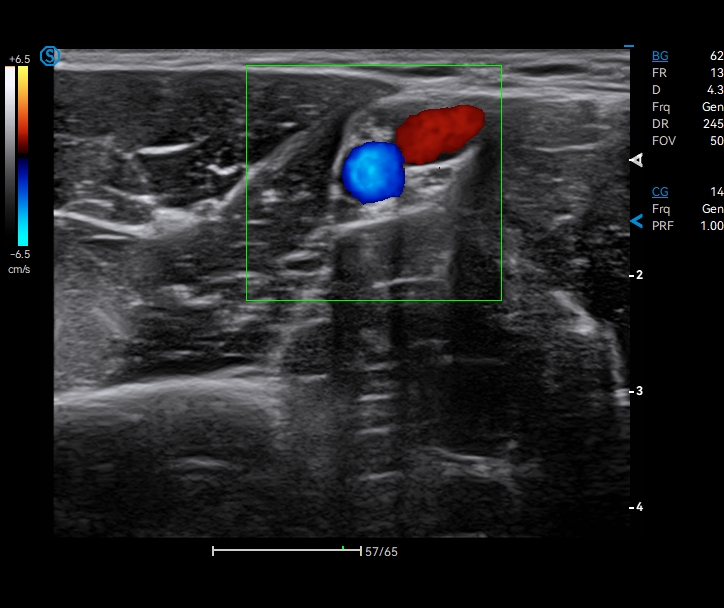
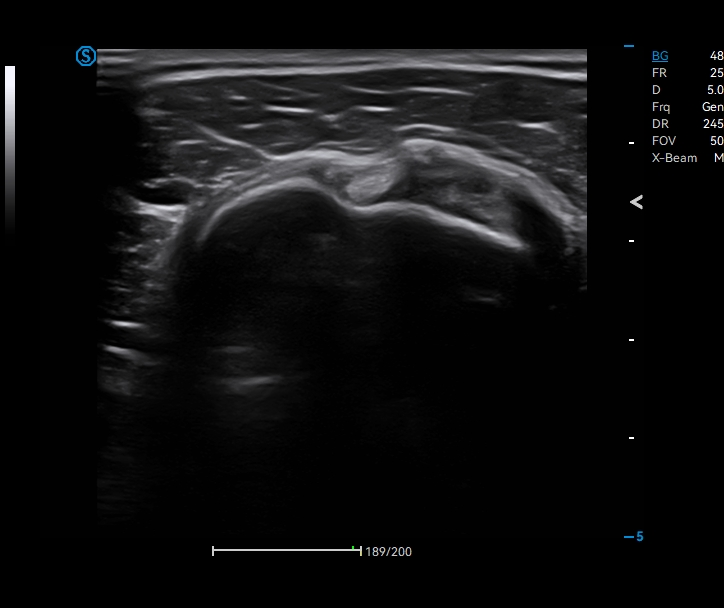
Apogee 2300 Pro
Powered by the robust Realview+ imaging platform, Apogee 2300 Pro delivers exceptional image quality and is especially excellent in urology and radiology applications. Its streamlined workflow ensures a smooth operation experience with the user-centered design concept. Choose Apogee 2300 Pro to upgrade your practice and upgrade your capabilities!
FeaturesFunctionGalleryDownload
Ergonomic design
· Foldable 15-inch HD screen
· Detachable lithium battery
· Compatible with the height-adjustable trolley
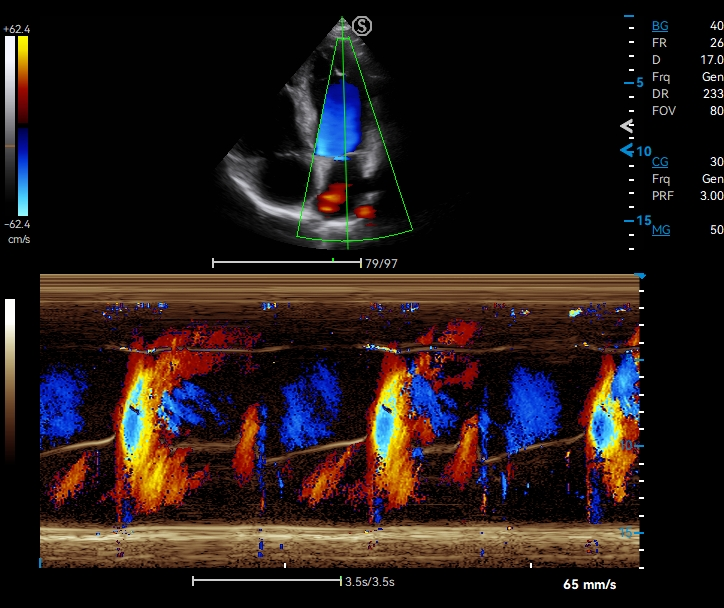
Upgraded Image Quality with Advanced Technology
· Pixel Echo Zone improves image uniformity
· Tailored Filter enhance S/N ratio to achieve higher image contrast
· S-Beam avoids image distortion
· Raw Data Processing enables flexible image adjustment
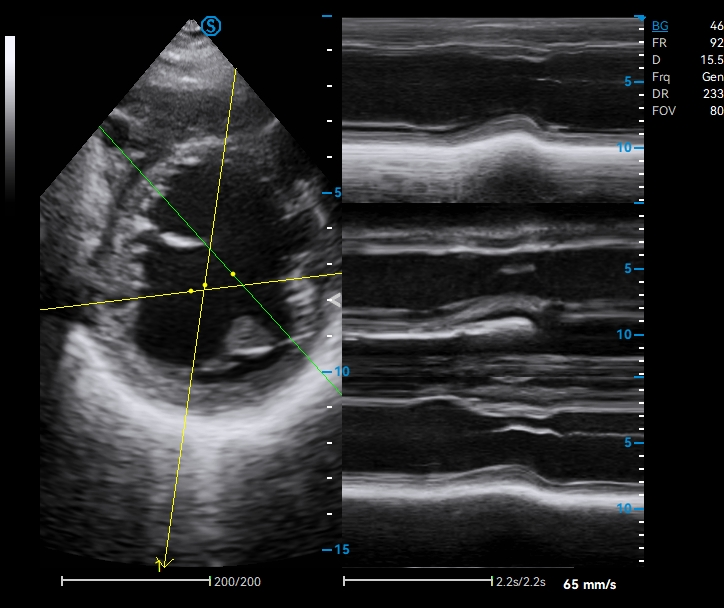
Upgraded Operation Experience with Streamlined Workflow
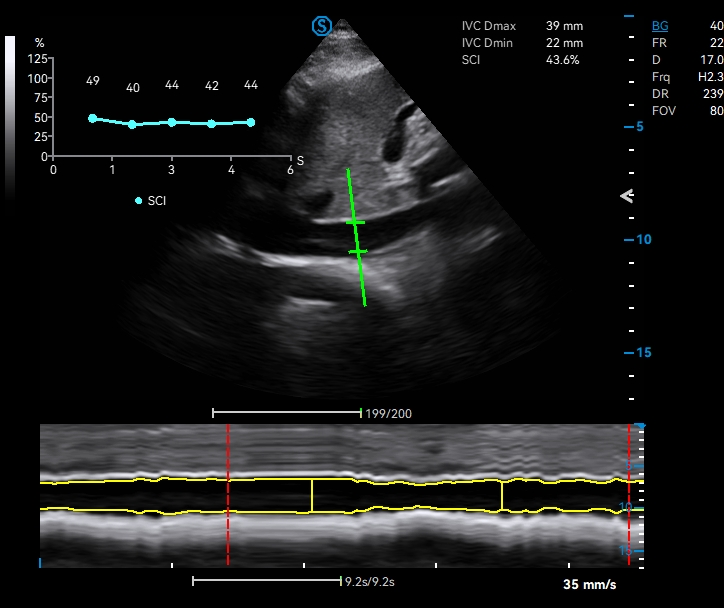
· Real-time Worksheet Display
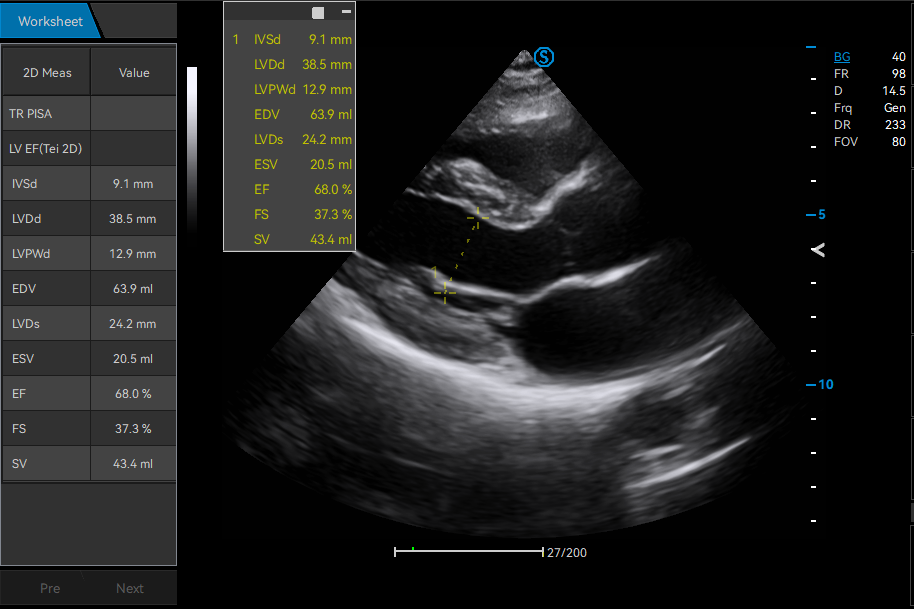
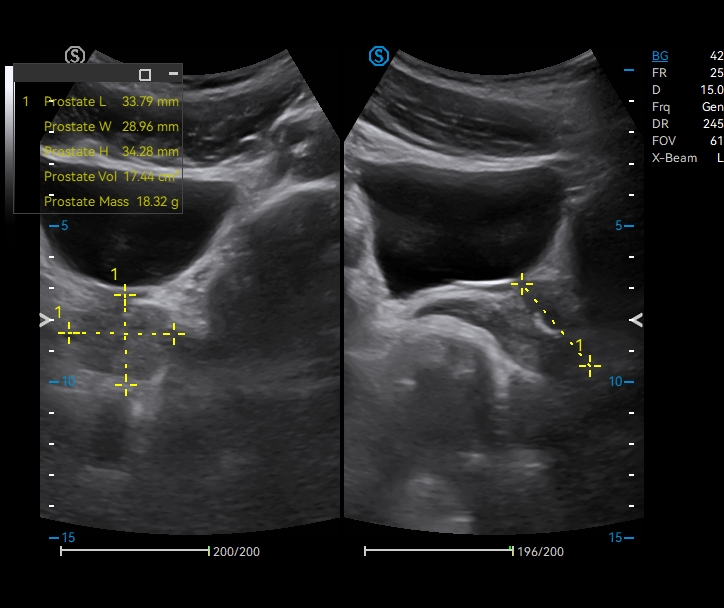
· Cross-mode Measurement

· Reversed data import
· Report Template Customization
· Exam Type Personalization
· Standby Mode
· Data Protection Mechanism
Urology
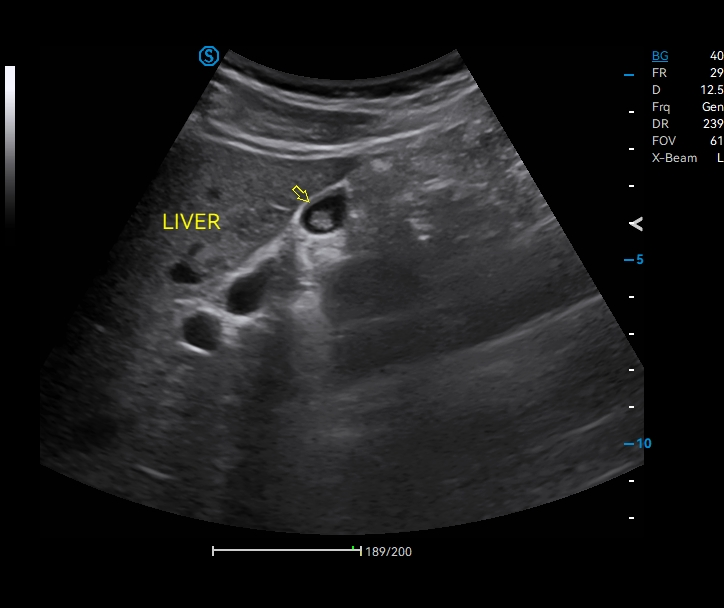
· In-plane Biopsy Guidance indicates the insertion path and needle tip position during biopsy operation.
· Out-of-plane Biopsy Guidance supports preset of needle size and insertion depth to minimize the risk of potential damage to surrounding tissues.
· Compatible with Bi-plane probe for efficient urology examination.
Bi-plane Dual Micro-convex Probe
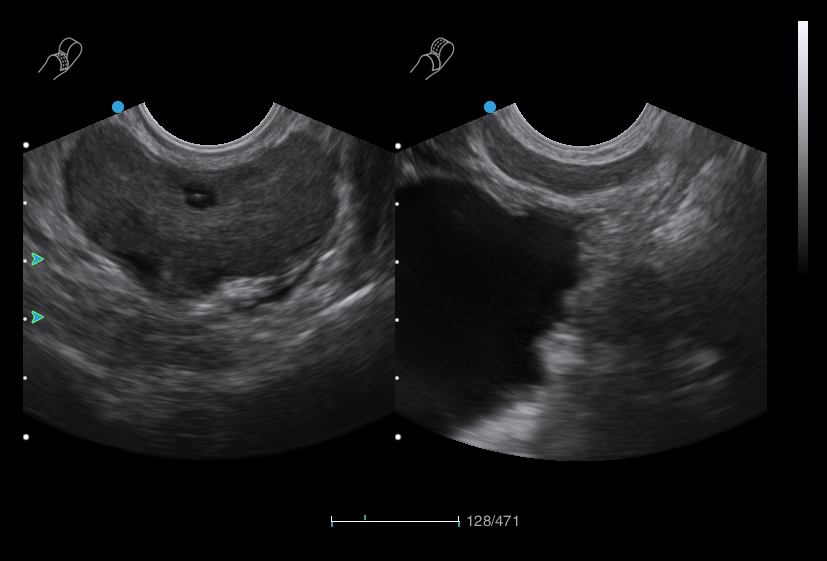
Bi-plane Linear and Micro-convex Probe
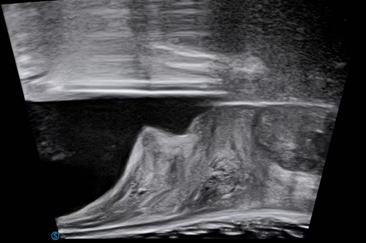
Radiology
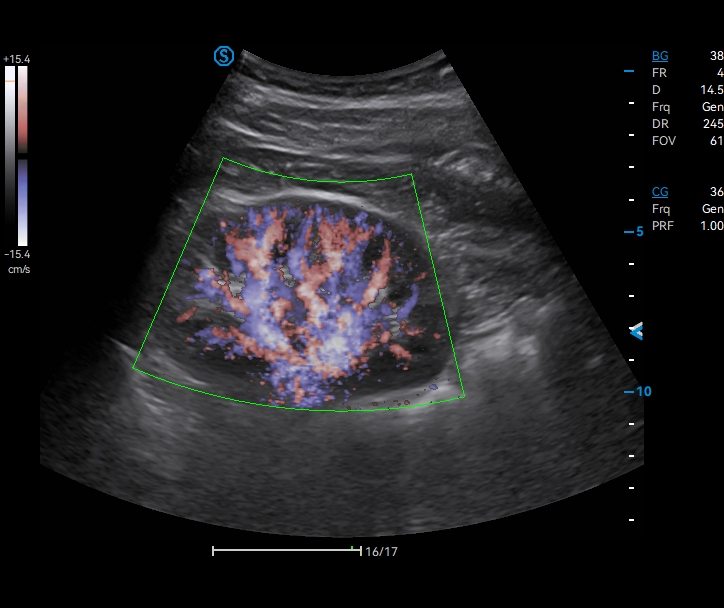
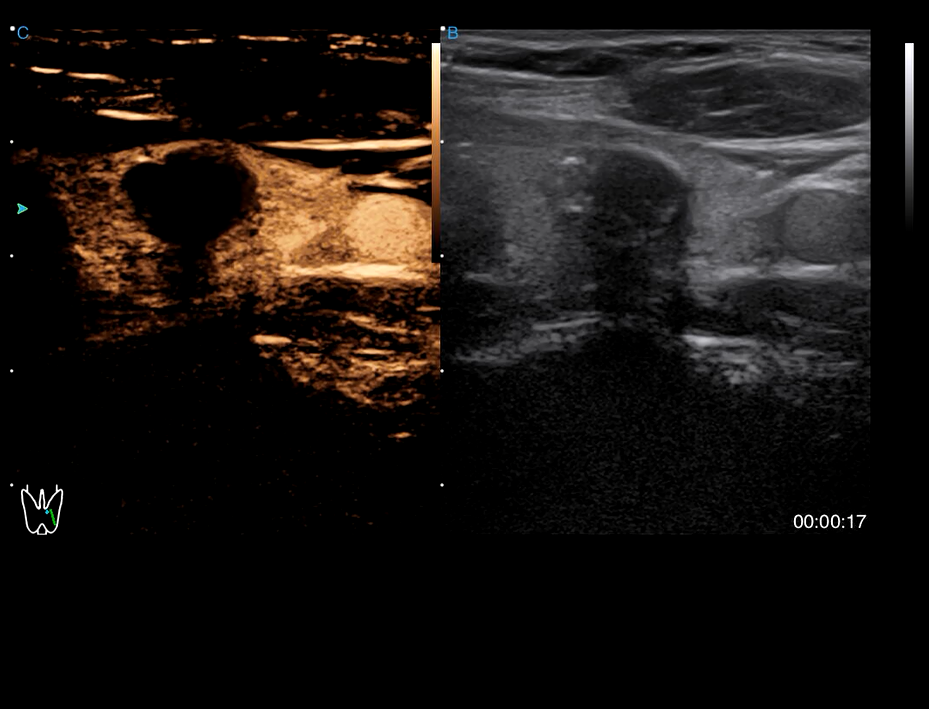
· CHI visualizes blood perfusion status in real-time with high image contrast to detect micro lesions and outline tumor.
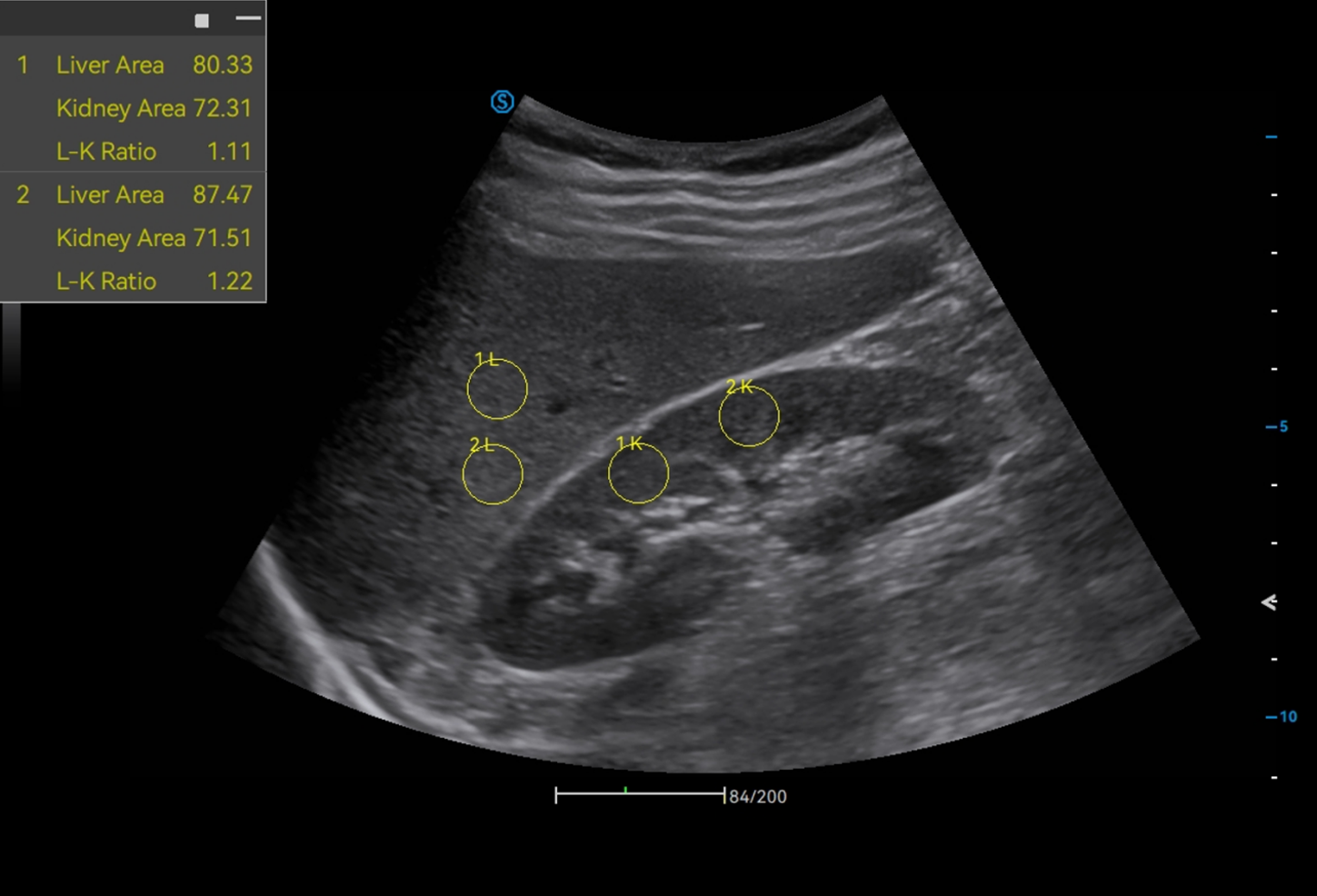
· HRI QA assesses hepatic steatosis by quantitative results with multiple sampling points available.
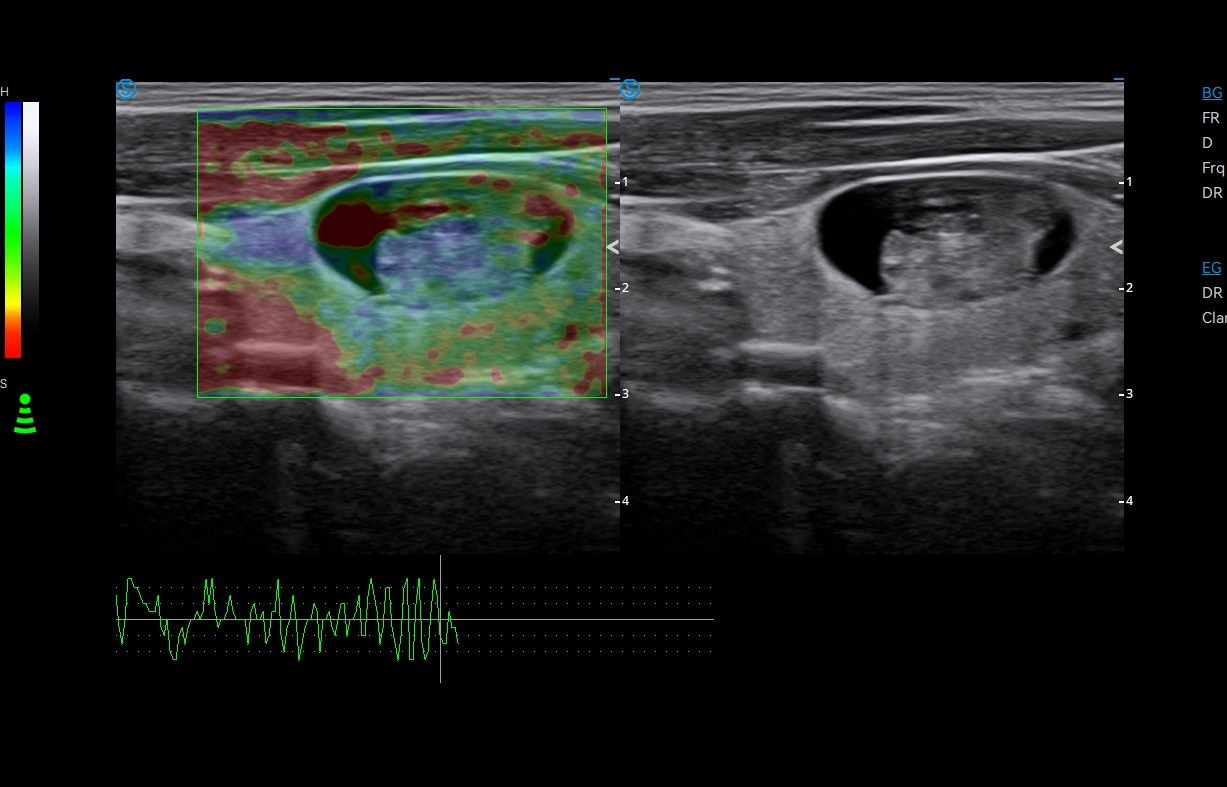
· Strain Elastography assists in analyzing tissue stiffness to detect potential abnormalities.
Due to differences in local regulatory requirements and sales policies, some product features may not be available in your region. This page is for reference only, and the actual situation of products shall prevail.